FDA Approves First Human Trial for Fully Implantable Speech Restoration Brain Chip
Paradromics, a U.S. neurotechnology startup, has received FDA approval for the first human trial of its fully implantable brain-computer interface designed to restore speech for people with paralysis. This milestone positions the Austin-based company at the forefront of neural technology innovation.
Key Takeaways
- First FDA-approved human trial for fully implantable speech restoration BCI
- Device converts neural activity into text or synthetic voice in real-time
- Initial study involves two participants, potentially expanding to ten
- Technology could help people with ALS, stroke, or spinal cord injuries
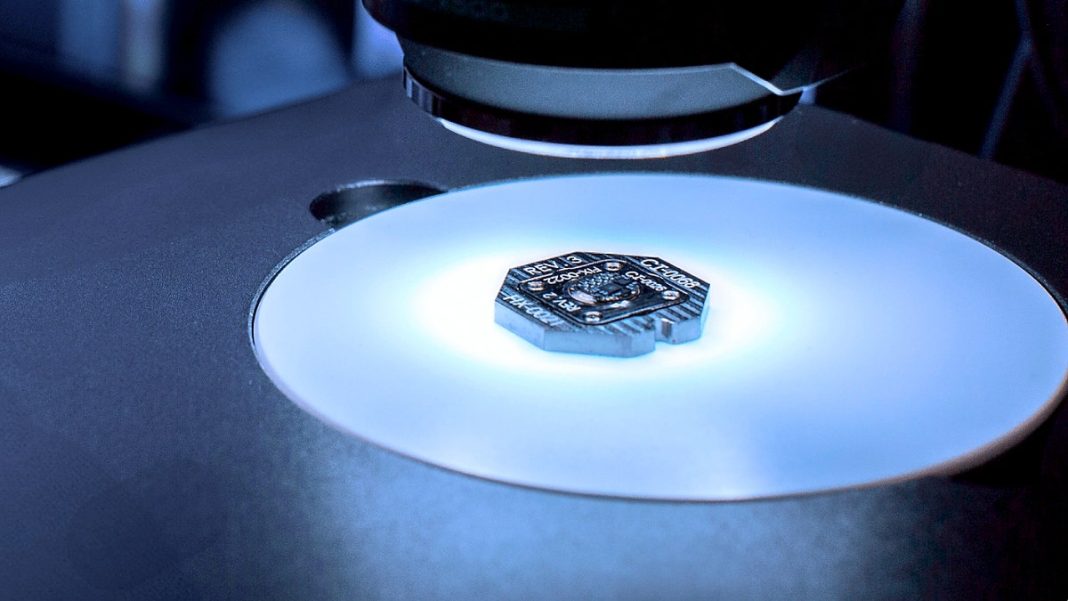
How the Brain Implant Works
The Connexus BCI features a titanium body with over 400 platinum-iridium electrodes, each thinner than a human hair. These electrodes record neural firing patterns from the motor cortex region controlling speech organs. The system uses wireless transceivers and inductive charging, with signals processed by advanced language models to generate text or synthetic voice based on the user’s past recordings.
Inside the Human Trial
The Connect-One Early Feasibility Study represents the first approved research exploring speech restoration with a fully implantable system. Participants receive a 7.5-millimeter electrode array implanted 1.5 millimeters into the motor cortex. During training, volunteers imagine speaking sentences while the device learns neural signatures of each sound.
This marks the first BCI trial formally targeting real-time synthetic voice generation. The study will also test whether the system can detect imagined hand movements for cursor control.
Communication is a fundamental human need. For people with severe motor impairment, the inability to express themselves with family and friends or request basic needs makes living difficult. The FDA approved clinical study for the Connexus Brain-Computer Interface is the first step toward a future where commercially available neurotech can restore the ability to naturally speak and seamlessly use a computer.
Competitive Landscape
Paradromics joins Synchron and Neuralink in the implanted BCI race. While Synchron uses a stent-like device recording broad neural patterns and Neuralink employs flexible threads for high-bandwidth signals, Paradromics occupies a middle ground with its fully implantable system that captures single-neuron detail while offering potential long-term stability.
Potential Impact
This breakthrough could significantly improve life for people who have lost speech ability due to ALS, stroke, or spinal cord injury. A system converting thought into speech could enable real-time communication and restore independence, while hands-free computer control could enhance daily living activities.
Paradromics is taking a careful but meaningful path toward practical BCI communication, setting the foundation for devices that may restore speech with natural flow and faster response times. As trials progress, this technology could transition from experimental to everyday use faster than anticipated.